Scientific
Approach
Our science is based on the premise that there is untapped biology at the interface of cancer and the immune system and an opportunity to select ideal anti-cancer drug targets from the list of “immuno-relevant” proteins upregulated in cancer.
Advancing the field of cancer target discovery
We believe that because the tumor must survive within the immune system, there are proteins that are upregulated for the specific purpose of circumventing immune surveillance and clearance. The importance of these upregulated proteins can only be found by deeply understanding the complex interactions of the immune system and cancer.
From this perspective, we identify Immuno-smart targets, representing a defined subset of the broader landscape of potential anti-cancer targets.
What we can now achieve
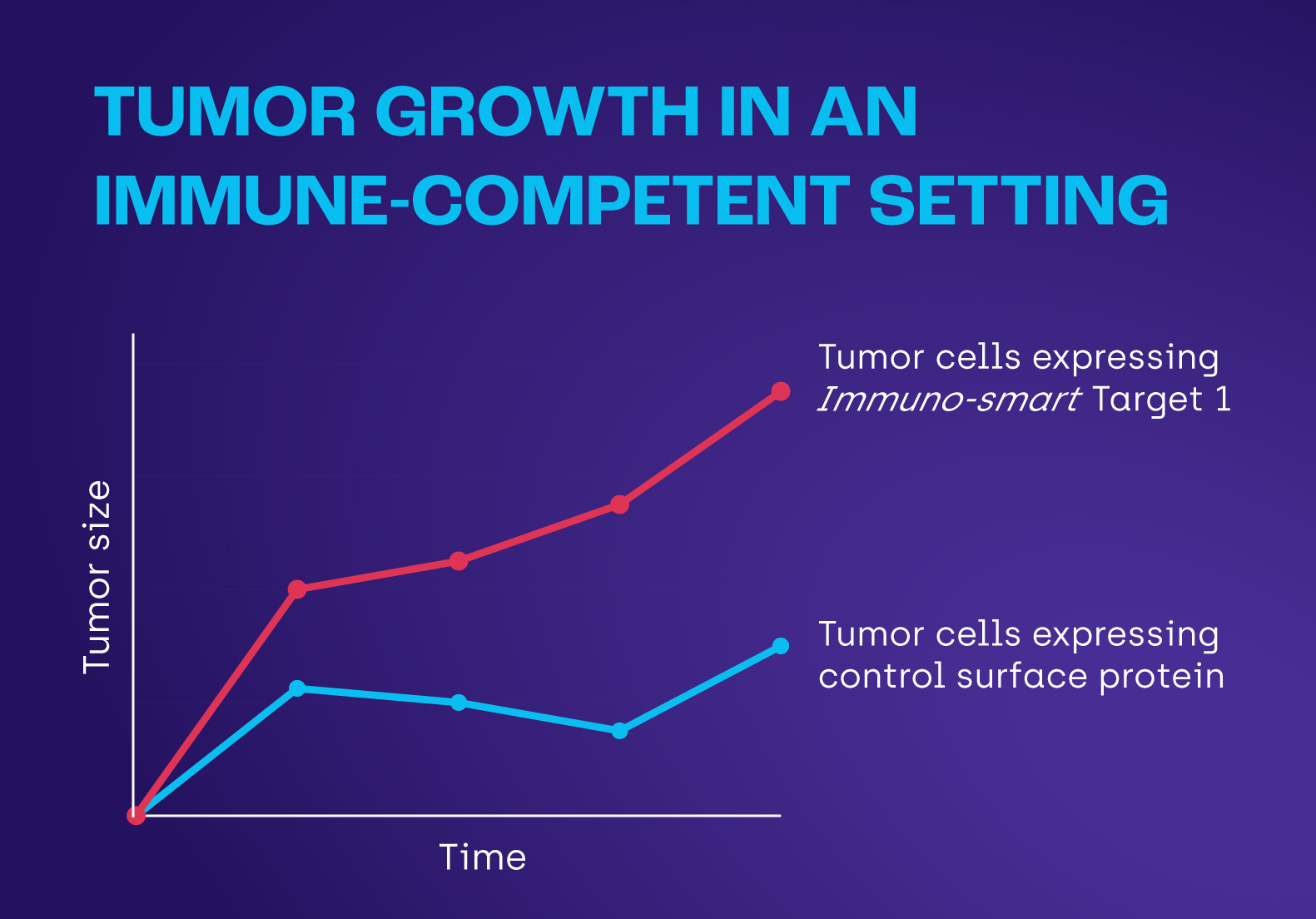
Our data show that our lead Immuno-smart target promotes tumor growth in the presence of the immune system. This unique function forms a foundational, bypass-resistant mechanism of immune evasion.
This essential presence at the tumor–immune interface makes Immuno-smart targets ideal docking sites for precision biologic medicines—including T-cell engagers, antibody–drug conjugates, and radioligand therapies—enabling a diversified pipeline from each Immuno-smart target.
platforms
Normunity was founded to be an integrated engine for novel anti-cancer drug discovery, seamlessly connecting groundbreaking research into the clinic. Together with the lab of Dr. Lieping Chen, a luminary in the field of cancer immunology, our drug discovery enterprise is designed to discover new therapies to reach more cancer patients by identifying and targeting novel mechanisms at the interface between the immune system and cancer.
AN INTEGRATED ENGINE FOR NOVEL CANCER DRUG DISCOVERY

Lieping Chen Lab DISCOVERY PLATFORM
Identify and validate novel targets
Deep target understanding
Generate hypothesis
Engineering platforms
Proposed biomarkers
Testing in animal models
Continuous enriching target understanding
Normunity Drug DEVELOPMENT
Develop novel medicines
Fully human monoclonal antibodies
Further animal testing
Chemistry, manufacturing and controls
Regulatory requirements
Clinical planning including Biomarker selection
Market strategy
Normunity Drug DEVELOPMENT
Our unique collaborative and iterative workflow is purpose-built to exchange scientific ideas, share key reagents and tools, and nurture basic science into clinical application. The Lieping Chen lab initiates hypothesis generation and building the essential assays to identify and validate novel targets. While the lab continues to interrogate the fundamental biology of targets, Normunity translates the target findings into drug candidates utilizing our deep experience in monoclonal antibody design and engineering and drug development.
UNCOVERING NOVEL MECHANISMS REQUIRES NEW PLATFORM CAPABILITIES
Because normal cancer-fighting immunity is disrupted by the cancer itself and involves multiple mechanisms, it difficult to study by conventional target-based methodologies. Our ability to target novel mechanisms is based on proprietary capabilities developed by the Lieping Chen lab using high throughput, physiologically relevant, phenotypic screening platforms to systematically interrogate the interactions between cancer and the immune system.
Key attributes of proprietary platforms enable us to reveal intersection points between cancer and the immune system
High
throughput
Simultaneous testing thousands of potential mechanisms
Unbiased
Interrogation of mechanisms without pre-supposed solution
Physiologically
Relevant
Utilize human cells and tissues
Rapid target
Deconvolution
Identify target that underlies observed phenotypic response
These proprietary platforms are building a comprehensive view of how the immune response malfunctions in cancer, revealing specific functional receptors and ligands that block the normal work of immune cells, including cytotoxic T-cells.